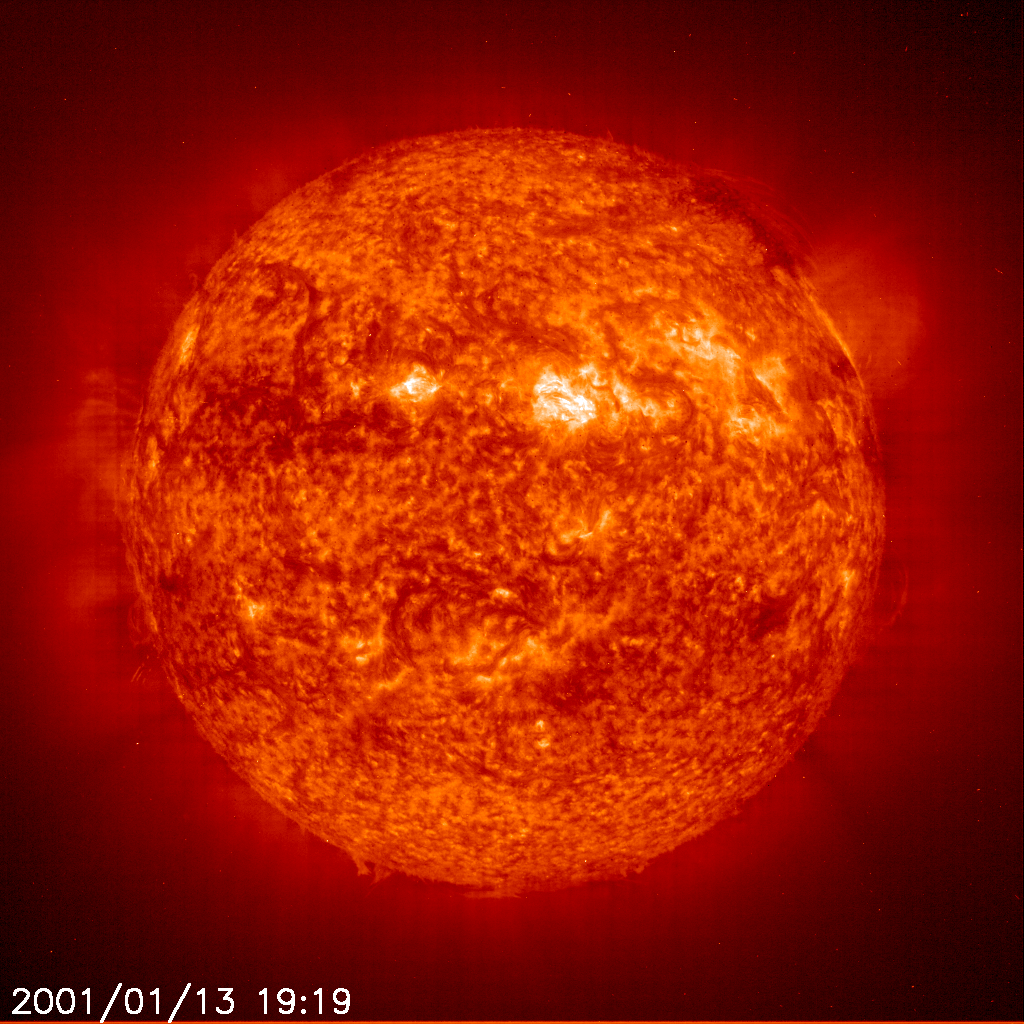
 This image of the active Sun was made using ultraviolet light emitted by ionized Helium atoms in the Solar chromosphere. Helium was first discovered in the Sun in 1868, its name fittingly derived from from the Greek word Helios, meaning Sun.
This image of the active Sun was made using ultraviolet light emitted by ionized Helium atoms in the Solar chromosphere. Helium was first discovered in the Sun in 1868, its name fittingly derived from from the Greek word Helios, meaning Sun.Out of the inert gases, I chose Helium because . . . because it's funnier than the other. Wikipedia says that helium and neon are the only true elemental inert gases, because they do not form any (known) true chemical compounds. That's very interesting. That means that helium is always helium, and neon is always neon. Helium is the second most abundant and second lightest element in the Universe and was one of the elements created in the Big Bang. In the modern Universe almost all new helium is created as a result of the nuclear fusion of hydrogen in stars. Because helium alone is less dense than atmospheric air, it will change the timbre (not pitch) of a person's voice when inhaled. However, inhaling it from a typical commercial source, such as that used to fill balloons, can be dangerous due to the number of contaminants that may be present. These could include trace amount of other gases, in addition to aerosolized lubricating oil. I wonder how many contaminants I've inhaled . . . lots, I'm sure.
No comments:
Post a Comment